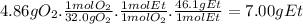Answer:
7.00 g
Step-by-step explanation:
There is some information missing. I think this is the complete question:
Wine goes bad soon after opening because the ethanol CH₃CH₂OH in it reacts with oxygen gas O₂ from the air to form water H₂O and acetic acid CH₃COOH, the main ingredient of vinegar. What mass of ethanol is consumed by the reaction of 4.86g of oxygen gas? Round your answer to 3 significant digits.
First, we have to write the balanced equation.
CH₃CH₂OH + O₂ ⇒ CH₃COOH + H₂O
Then, we can establish the following relations:
- The molar mass of O₂ is 32.0 g/mol
- The molar ratio of O₂ to CH₃CH₂OH is 1:1
- The molar mass of ethanol is 46.1 g/mol.
The mass of ethanol that reacts with 4.86 g of oxygen is: