Answer:
9.4 ppmv
Step-by-step explanation:
Parts per million by volume means milligrams of solute per liter of solution. Then, we have to find the mass of the solvent in mg (which in this context is the solute) and the volume of the room in L (which in this context is the volume of the solution).
For the solute, we know:
- The volume is 404 mL.
- The specific gravity is 1.37, which means that the density is 1.37 g/mL.
- 1 g = 1000 mg.
The mass of the solute is:
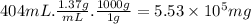
For the room, we know:
- The dimensions are 21 ft × 10 ft × 10 ft.
- 1 ft³ = 28.3 L
The volume of the room is:
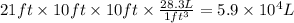
The resulting concentration in ppmv is:
