Answer:
The balanced single displacement reaction between aluminum and copper sulphate is as follows.
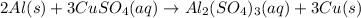
Step-by-step explanation:
The given ions are as follows.
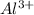 - Aluminium ion and copper sulfate ion.
- Aluminium ion and copper sulfate ion.
Single displacement reaction occurs one element replaces another element.
Hence, the balanced chemical reaction is as follows.
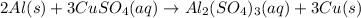
Oxidation half reaction:
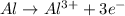
Aluminium loses three electrons.
Reduction half reaction:
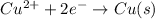
Copper gain three electrons.