Answer:
1270 Psi
Step-by-step explanation:
We are given;
- Pressure in the tank, P₁ as 422 psi
- Temperature in the tank, T₁ as 20.0 K
- New temperature in the tank, T₂ as 60.0 K
We are required to determine the pressure at 60.0 K
- We are going to use the pressure law;
- According to the pressure law, the pressure of a gas is directly proportional to the temperature at constant volume.
- That is;
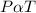
- Thus,
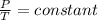
- At different pressures and temperature;
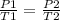
- Thus, rearranging the formula;
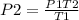
Hence;
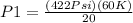
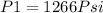
Therefore;
P₂=1266 Psi
= 1270 Psi
Thus, the pressure at 60.0 K is 1270 Psi