Answer:
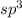 on N overlaps with 1s on H.
on N overlaps with 1s on H.
Step-by-step explanation:
Hybridization of central atom generally can be found by finding the hybridization number.Hybridization number is the sum of number of lone pairs and number of sigma bonds.
If hybridization number is 2 then the central atom is sp hybridized.
If it is 3 it is
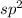 hybridized.
hybridized.
If it is 4 it is
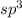 hybridized and so on.
hybridized and so on.
Here in
 molecule there are 3 sigma bonds and one lone pair(see the figure attached).
molecule there are 3 sigma bonds and one lone pair(see the figure attached).
Hence hybridization number is 4 and hence it is s
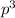 hybridized.
hybridized.
And in case of hydrogen atom the only electron of it resides in the 1s orbital.
Therefore
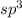 on N overlaps with 1s on H.
on N overlaps with 1s on H.