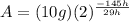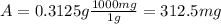Answer: d) 312.5 mg
Step-by-step explanation:
This problem can be solved using the Radioactive Half Life Formula:
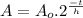
Where:
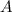 is the remaining amount of Actinium-226
is the remaining amount of Actinium-226
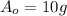 is the initial amount of Actinium-226
is the initial amount of Actinium-226
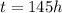 is the time elapsed
is the time elapsed
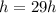 is the half life of Actinium-226
is the half life of Actinium-226
Knowing this, let's find
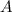 :
: