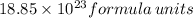Answer:
1)Molar mass of calcium citrate is 498.46g/mol
4) Mass of
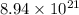 molecules of ethanol is
molecules of ethanol is
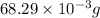
5)926 gm of
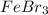 has
has
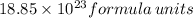
Step-by-step explanation:
1)
The molecular formula of Calcium citrate-
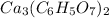
Let's calculate the molar mass.
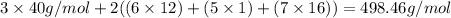
Therefore, molar mass of calcium citrate is 498.46g/mol
4)
One mole of molecule has
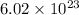 molecules.
molecules.
Molar mass of ethanol = 46.07 g/mol
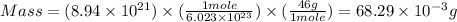
Therefore, mass of
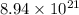 molecules of ethanol is
molecules of ethanol is
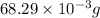
5)
Molar mass of
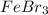 = 295.56g/mol
= 295.56g/mol
One mole has
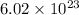 formula units.
formula units.
Given mass = 926 g
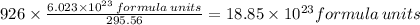
Therefore, 926 gm of
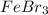 has
has