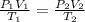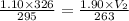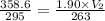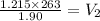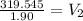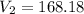Answer:
168.26
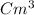 is the volume of the cooled gas.
is the volume of the cooled gas.
Step-by-step explanation:
From “Charles law” for given “mass of gas” the “volume of given mass of a gas” is directly proportional to its “temperature” when expanded.
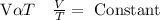
From “Boyle’s law” for given “mass of gas” the “volume of given mass of a gas” is inversely proportional to “pressure of given mass of a gas” when expanded.
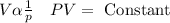
From “Gay-Lussac law” for given “mass of gas” the “pressure of given mass of a gas” is directly proportional to “temperature of given mass of a gas” when expanded.
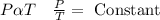
By combining all three gas laws “Charles law”, “Boyle’s law” and “Gay-Lussac law” we know that
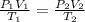
Given that,
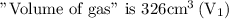
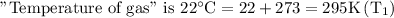
After the gas is cooled the “Pressure of gas” is 1.90 atmosphere
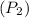
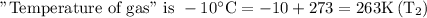
We need to find the “Volume of gas” after cooling.
Substitute the given values in the