Answer:
d) 2v
Step-by-step explanation:
Since, root mean square speed of a molecule,
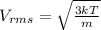
Where,
k = Boltzmann constant,
T = temperature of gas,
m = mass of molecule,
Also, the temperature of a gas,
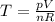
Where,
p = pressure of the gas,
V = volume,
n = number of moles of gas,
R = universal gas constant,
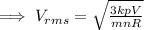
If V = 2V and p = 2P
Then,
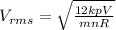
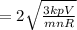
Hence, if rms speed of a gas molecule under initial conditions is v then rms speed of a molecule will be 2v
i.e. option d is correct.