Answer:
The de Broglie wavelength associated with a baseball is
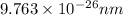 .
.
Step-by-step explanation:
De-Broglie wavelength is calculated by using the formula:
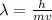 .....(1)
.....(1)
where,
 = wavelength of particle
= wavelength of particle
h = Planck's constant =
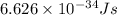
m = mass of particle
v = velocity of particle
We have :
m = 220 g =0.220 kg
velocity of the baseball , v= 69 mile/h
1 mile = 1609.34 m , 1 hour = 3600 s
v =
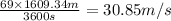
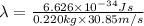
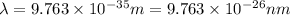
(
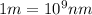 )
)
The de Broglie wavelength associated with a baseball is
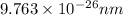 .
.