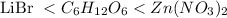Answer: The order of increasing boiling points follows:
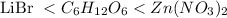
Step-by-step explanation:
The expression of elevation in boiling point is given as:
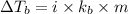
where,
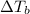 = Elevation in boiling point
= Elevation in boiling point
i = Van't Hoff factor
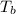 = change in boiling point
= change in boiling point
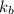 = boiling point constant
= boiling point constant
m = molality
For the given options:
- Option 1: 0.12 m
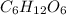
Value of i = 1 (for non-electrolytes)
So, molal concentration will be =
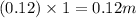
Value of i = 2
So, molal concentration will be =
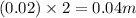
- Option 3: 0.05 m
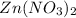
Value of i = 3
So, molal concentration will be =
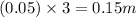
As, the molal concentration of
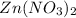 is the highest, so its boiling point will be the highest.
is the highest, so its boiling point will be the highest.
Hence, the order of increasing boiling points follows: