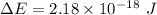Answer:
B.
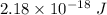
Step-by-step explanation:
Ionization energy is the minimum amount of energy which is required to knock out the loosely bound valence electron from the isolated gaseous atom.
The expression for the energy of an electron in the nth orbit is:-
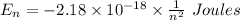
For transitions:
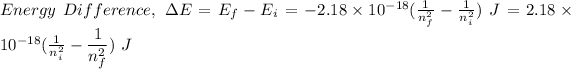
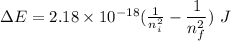
Given,
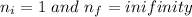

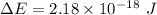
Ionization energy,