Answer:
*
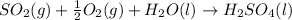
*
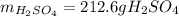
*
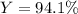
Step-by-step explanation:
Hello,
In this case, the balanced chemical reaction is:
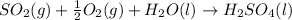
Now, by means of the Avogadro's law, it is possible to compute the moles of sulfur dioxide as shown below:
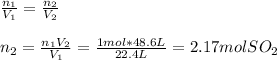
Thus, by stoichiometry, the theoretical yield of sulfuric acid in grams is:
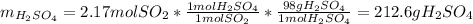
Finally, the percent yield results:
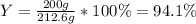
Best regards.