Answer:
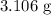
Step-by-step explanation:
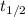 = Half-life of carbon = 5700 years
= Half-life of carbon = 5700 years
t = Time at which the remaining mass is to be found = 10400 years
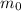 = Initial mass of carbon = 11 g
= Initial mass of carbon = 11 g
Decay constant is given by
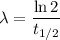
Amount of mass remaining is given by
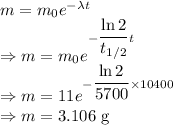
The amount of the substance that remains after 10400 years is
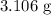 .
.