Answer:
B. CA, 14
Step-by-step explanation:
Atoms of elements contain small particles known as electrons, neutrons, and protons. The nucleus of an atom is made up of neutrons and protons which are at the center of the atom. Electrons on the other hand surrounds the nucleus. Electron has negative charge while proton has a positive charge. The number of neutrons is equivalent to the number of protons . In addition, the number of protons is equal to mass number minus the number of electrons.
For the compound
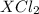 , it can be broken down into
, it can be broken down into
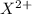 and
and
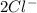 . Its ion has a mass of 34 and 18 electrons which means it has already lost 2 electrons.
. Its ion has a mass of 34 and 18 electrons which means it has already lost 2 electrons.
Therefore:
For the given element, the number of electrons is 18+2 = 20 electrons.
The number of protons = 34 - 20 = 14.
And the number of neutrons is 14.
Only option B has the correct answer.