The mixture would not need to have a certain ratio of hydrogen atoms to oxygen atoms is the difference between mixture of hydrogen and oxygen differ from the compound known as water.
Option B
Step-by-step explanation:
Water molecule contains one oxygen atom and two hydrogen atoms which reacted chemically. This means once they go through the chemical reaction process, they lose their individual properties and obtain new properties of water.
If oxygen and hydrogen form a mixture, they retain their individual properties, they don't need any ratio or and most importantly there is no chemical reaction taking place. Hydrogen Peroxide
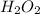 isn't a mixture but a compound formed when two hydrogen atoms react with two oxygen atoms. The most important factor distinguishing between compound and mixture is a chemical reaction.
isn't a mixture but a compound formed when two hydrogen atoms react with two oxygen atoms. The most important factor distinguishing between compound and mixture is a chemical reaction.