Answer: The value of q for the reaction will be -100.1 kJ
Step-by-step explanation:
To calculate the number of moles, we use the equation:

Given mass of methane = 1.80 g
Molar mass of methane = 16 g/mol
Putting values in above equation, we get:
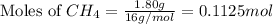
For the given chemical reaction:
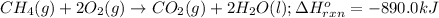
By Stoichiometry of the reaction:
When 1 mole of methane reacts, the heat released is 890.0 kJ
So, when 0.1125 moles of methane will react, the heat released will be
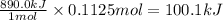
Sign convention of heat (q):
When heat is absorbed, the sign of heat is taken to be positive and when heat is released, the sign of heat is taken to be negative.
Hence, the value of q for the reaction will be -100.1 kJ