Answer:
The chemical shift (δ) for CHBr₃ proton = 6.88 ppm
Step-by-step explanation:
In NMR spectroscopy, Chemical shift (δ) is expressed in parts per million (ppm) and is given by the equation:
 ....equation (1)
....equation (1)
Given: Observed frequency: ν₁ = 2065 Hz,
Spectrometer frequency: ν'₁ = 300 MHz, ν'₂ = 200 MHz
To calculate the chemical shift (δ) for the given CHBr₃ proton, we use the equation (1)
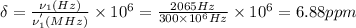
Since in NMR spectroscopy, chemical shift is a field independent scaling. Thus the value of the chemical shift of a given proton, such as CHBr₃ proton, is independent of the magnetic field strength of the spectrometer.
So the value of chemical shift of a given proton remains same when measured with a 300 MHz and 200 MHz NMR spectrometer.
Therefore, the chemical shift (δ) for CHBr₃ proton = 6.88 ppm