Answer:
0.3 mol/L is the concentration of the diluted solution in the 50 mL volumetric flask.
Step-by-step explanation:
We have 100 mL of solution with a concentration of 1.50 mol/L.
Molarity of the solution before dilution =
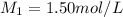
Volume 1.50 mol/L solution added in 50 mL volumetric flask =
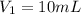
Molarity of the solution after dilution =
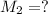
Volume of solution after dilution =
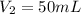
 (dilution)
(dilution)
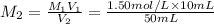
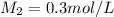
0.3 mol/L is the concentration of the diluted solution in the 50 mL volumetric flask.