Answer:
20 molecules of oxygen gas remains after the reaction.
Step-by-step explanation:
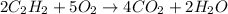
Molecules of ethyne = 52
Molecules of oxygen gas = 150
According to reaction, 2 molecules of ethyne reacts with 5 molecules of oxygen gas.
Then 52 molecules of ethyne will react with:
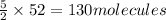 of oxygen gas.
of oxygen gas.
As we can see that we have 150 molecules of oxygen gas, but 52 molecules of ethyne will react with 130 molecules of oxygen gas. So, this means that ethyne is a limiting reagent and oxygen gas is an excessive reagent.
Remaining molecules of recessive reagent = 150 - 130 = 20
20 molecules of oxygen gas remains after the reaction.