Answer: The true statement is iron can reduce
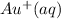 to gold metal
to gold metal
Step-by-step explanation:
Single displacement reaction is defined as the reaction in which more reactive element displaces a less reactive element from its chemical reaction.
The reactivity of metal is determined by a series known as reactivity series. The metals lying above in the series are more reactive than the metals which lie below in the series.

Metal A is more reactive than metal B.
We are given:
Iron can reduce copper, silver can reduce gold, sodium can reduce iron and copper can reduce silver metal.
The increasing order of reactivity thus follows:
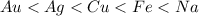
where, sodium is most reactive and gold is least reactive
For the given options:
Option 1: Copper cannot easily reduce sodium ion to sodium metal because it is less reactive.
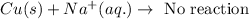
Option 2: Iron cant easily reduce gold ion to gold metal because it is more reactive.
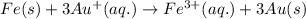
Option 3: Silver cannot easily reduce iron ion to iron metal because it is less reactive.
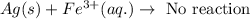
Hence, the true statement is iron can reduce
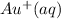 to gold metal
to gold metal