Answer: Yes, a wavelength of 230 nm can photo ionize O2.
Step-by-step explanation:
Wavelength, λ = 230 nm = 230 ×
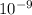
Bond disassociation energy per mole = 495 kJ/mol
Bond disassociation energy per molecule =
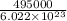
= 8.22×

Wavelength associated with this bond energy is, =
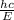
=
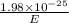
= 242 nm
Therefore, any wavelength shorter than 242 nm can photo ionize O2.