Answer: Option (a) is the correct answer.
Step-by-step explanation:
Sodium bromide (NaBr) is an ionic salt as it is formed by transfer of an electron from sodium to bromine atom.
Whereas
 , HCN,
, HCN,
 and
and
 are all covalent compounds. And, all of them exists as a gas at room temperature.
are all covalent compounds. And, all of them exists as a gas at room temperature.
Also according to rate of diffusion law, rate of diffusion is inversely proportional to square root of molecular weight of gas.
Mathematically, Rate of diffusion =
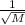
Out of the given options NaBr has the highest molecular weight which is 102.894 g/mol.
Thus, we can conclude that NaBr is most likely not a gas at room temperature.