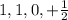Answer:
The incorrect quantum numbers are
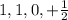
Step-by-step explanation:
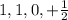
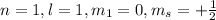
It is not a valid set of quantum number, "l" value cannot be taken from "n" value.
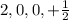
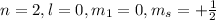
This set of quantum number represents second energy level.
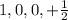
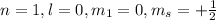
This set of quantum number represents first energy level.
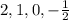
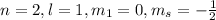
This set of quantum number represents 2p energy level.
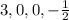
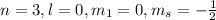
This set of quantum number represents third energy level.
Therefore, The incorrect quantum numbers are