The question is incomplete, here is a complete question.
Party stores sell small tanks containing 41 g of helium gas.
If you use such a tank to fill 0.020 m³ foil balloons (which don't stretch, and so have an internal pressure that is very close to atmospheric pressure), how many balloons can you expect to fill? Assume the temperature is 20°C.
Answer : The number of balloon filled can be, 12 balloons.
Explanation :
First we have to calculate the number of moles of gas in balloon.
Using ideal gas equation:

where,
P = pressure of gas = 1 atm (atmospheric pressure)
V = volume of gas =
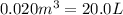
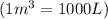
T = temperature of gas =
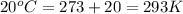
n = number of moles of gas = ?
R = gas constant = 0.0821 L.atm/mol.K
Now put all the given values in the ideal gas equation, we get:
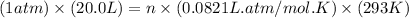
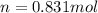
Thus, the number of moles of gas in balloon is 0.831 mol per balloon.
Now we have to calculate the mass of gas in balloon.
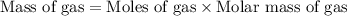
Molar mass of He gas = 4 g/mole
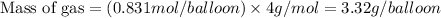
Now we have to calculate the number of balloon can be filled.
Number of balloon filled =
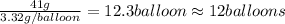
Therefore, the number of balloon filled can be, 12 balloons.