Step-by-step explanation:
The given data is as follows.
mass of ice = 8.8 g, mass of water = 250 g
It is known that latent heat of fusion of ice is 333 J/g. Then, heat energy of ice will be calculated as follows.
Q =
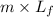
=
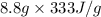
= 2930.4 J
As heat absorbed by water = heat released by ice
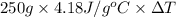 = 2930.4 J
= 2930.4 J
 = 2.8°C
= 2.8°C
thus, we can conclude that the temperature change in the water upon the complete melting of the ice is 2.8°C.