Answer:
(a) The final pressure of the sample becomes one-fourth of the original pressure.
(b) The pressure of the sample remains unchanged.
(c) The final pressure of the sample becomes four times of the original pressure.
Step-by-step explanation:
(a)
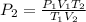
The volume of sample doubled and kelvin temperature halved.
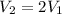
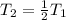
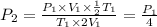
Therefore, the final pressure of the sample becomes one-fourth of the original pressure.
(b)
Volume and temperature of sample doubled.
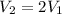

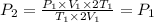
Therefore, the pressure of the sample unchanged.
(c)
Volume of sample halved and temperature double.
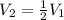

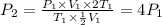
Therefore, the pressure of the sample becomes four times of the original pressure.