Answer:
Number of produced moles of carbondioxide is 0.113 mol.
Step-by-step explanation:
Excess of sulphuric acid is present.And the sodium carbonate is completely consumed.
Molar mass of sodium carbonate = 105.99g/mol
Given mass of sodium carbonate =12 g/mol
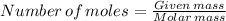
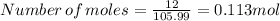
one mole of sodium carbonate produce one mole of carbondioxide.
0.113 mole of sodium carbonate produce 0.113 mole of carbondioxide.
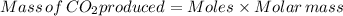
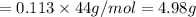
Therefore, 0.113 mol of carbondioxide produced in flask 7.