Answer:
1368 grams.
Step-by-step explanation:
Molarity is defined as number of moles of solute present in one litre of solution. 1-M means 1 mole of solute is present in 1 litre of solution.
4-M solution means 4 moles of solute is present in 1 litre of solution.
Molar mass of
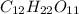 =
=
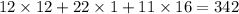 grams.
grams.
as molar mass of carbon,hydrogen and oxygen atoms are 12,1 and 16 grams respectively.
Since molar mass of
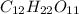 is 342 grams,
is 342 grams,
1 mole of it weighs 342 grams and 4 moles of it weighs
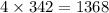 grams.
grams.
Hence we need 1368 grams to make a 4-M solution of
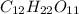 .
.