Answer:
The balanced chemical reaction is as follows.
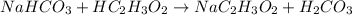
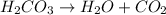
Step-by-step explanation:
Balanced chemical reaction has equal number of atoms in reactant side and product side.
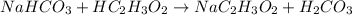
Reactant side Product side
Number of sodium atoms -1 Number of sodium atoms -1
Number of hydrogen atoms -5 Number of hydrogen atoms -5
Number of carbon atoms -3 Number of carbon atoms -3
Number of oxygen atoms-5 Number of oxygen atoms-5
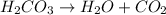
Reactant side Product side.
Number of hydrogen atoms -2 Number of hydrogen atoms -2
Number of carbon atoms -1 Number of carbon atoms -1
Number of oxygen atoms -3 Number of oxygen atoms -3