Answer:
The correct answer is :'Salt water and vinegar have equal molarities.'.
Step-by-step explanation:
Molarity of the solution is defined as moles of compound present in the 1 liter volume of the solution.
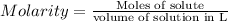
We are given with 3 Molar solution of vinegar which means that 3 moles of acetic acid are present in 1 Liter solution of vinegar.
Also salt solution with 3 Molar concentration means that 3 moles of salt are o present in 1 Liter salt solution.
Molarity of acetic acid = Molarity of salt solution = 3 M