Answer:
2 moles of ammonium ions required to combine with 1 mole of oxalate ion to form ammonium oxalate.
Step-by-step explanation:
Ammonium cation=
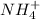
Oxalate anions =
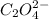
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound as shown in the image attached.
The ionic compound that formed when ammonium ion and oxalate ions combine is ammonium oxalate.
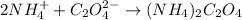
2 moles of ammonium ions required to combine with 1 mole of oxalate ion to form ammonium oxalate.