Answer:
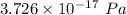

844.58565402 km
Step-by-step explanation:
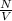 = Density of atoms =
= Density of atoms =
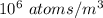
n = Amount of substance = 1 mol
V = Volume
R = Gas constant = 8.314 J/mol K
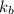 = Boltzmann constant =
= Boltzmann constant =
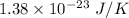
T = Temprature = 2.7 K
L = Side of cube
From ideal gas law we have the relation
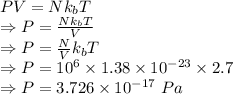
The pressure is
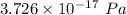
From ideal gas law
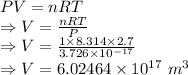
The volume is

Volume is given by
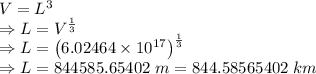
The length of the side of the cube is 844.58565402 km