Answer:
The molar mass of the compound is:- 168.82 g/mol
The molar mass of the gas is:- 16.38 g/mol
Step-by-step explanation:
(a)
Using ideal gas equation as:

where,
P is the pressure
V is the volume
n is the number of moles
T is the temperature
R is Gas constant having value = 0.0821 L.atm/K.mol
Also,
Moles = mass (m) / Molar mass (M)
Density (d) = Mass (m) / Volume (V)
So, the ideal gas equation can be written as:
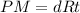
Given that:-
Pressure = 20 kPa = 20000 Pa
The expression for the conversion of pressure in Pascal to pressure in atm is shown below:
P (Pa) =
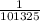 P (atm)
P (atm)
20000 Pa =
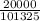 atm
atm
Pressure = 0.1974 atm
Temperature = 330 K
d = 1.23 kg/m³ = 1.23 g/L
Molar mass = ?
Applying the equation as:
0.1974 atm × M = 1.23 g/L × 0.0821 L.atm/K.mol × 330 K
⇒M = 168.82 g/mol
The molar mass of the compound is:- 168.82 g/mol
(b)
Given that:
Pressure = 152 Torr
Temperature = 298 K
Volume = 250 cm³ = 0.25 L
Using ideal gas equation as:

R =
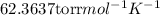
Applying the equation as:
152 Torr × 0.25 L = n × 62.3637 L.torr/K.mol × 298 K
⇒n = 0.002045 moles
Given that :
Mass of the gas = 33.5 mg = 0.0335 g
Molar mass = ?
The formula for the calculation of moles is shown below:

Thus,
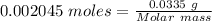
The molar mass of the gas is:- 16.38 g/mol