Answer:
(a) 45.17×10^-14 kg/s
(b) since the amount of helium escaped due to diffusion is insignificant, the final pressure drop in the tank remains the same as the initial pressure 500kPa.
Explanation:
Helium gas at temperature T=293k
Helium gas at pressure P= 500kPa
The inner diameter of spherical tank is
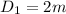
The inner radius of spherical tank is :
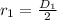
=
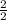
=1m
Thickness of the container r = 1cm =0.01m
Outer radius of the spherical tank is ;
t =

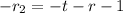
multiplying through with (-) we have ;
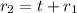
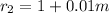
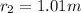
From table of binary diffusion coefficients of solids, the diffusion coefficients of helium in silica is noted as
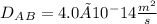
From table molar mass and gas constant, the molecular weight of helium is:
M = 4.003kg/kmol
The solubility of helium in fused silica is determined from Table of Solubility of selected gases and soilids.
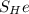 = 0.00045 kmol/m³. bar
= 0.00045 kmol/m³. bar
Considering total molar concentration as constant, the molar concentration of helium inside the container is determined as
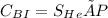
= 0.00045kmol/m³. bar × (5)
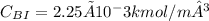
From one dimensional mass transfer through spherical layers is expressed as:
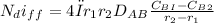
substituting all the values in the above relation, we have;
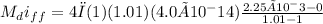
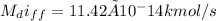
(a) The mass flow rate is expressed as
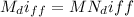
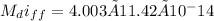
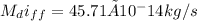
(b) The pressure drop in the tank after a week;
For one week the mass flow rate of helium is
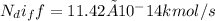
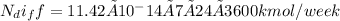
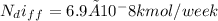
The volume of the spherical tank is

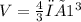
V = 4.189m³
The initial mass of helium in the sphere is determined from the ideal gas equation:
PV=NRT
where R is the universal gas constant and its value is R = 8.314 KJ/Kmol.k
N= PV/RT
N= 500 × 4.189/ 8.314 × 293
N= 0.86kmol
The number of moles of helium gas remaining in the tank after one week is:
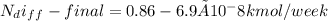
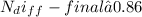
therefore, since the amount of helium escaped due to diffusion is insignificant, the final pressure drop in the tank remains the same as the initial pressure 500kPa.