Answer: The value of
 for the reaction will be -537 kJ
for the reaction will be -537 kJ
Step-by-step explanation:
To calculate the number of moles, we use the equation:
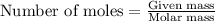
Given mass of hydrogen gas = 0.647 g
Molar mass of hydrogen gas = 2 g/mol
Putting values in above equation, we get:
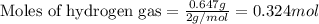
We are given:
Energy released for 0.324 moles of hydrogen reacted is 174 kJ
For the given chemical reaction:
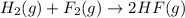
By Stoichiometry of the reaction:
When 0.324 moles of hydrogen gas is reacted, the energy evolved is 174 kJ
So, when 1 mole of hydrogen gas will react, the energy evolved will be =
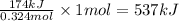
Sign convention of heat:
When heat is absorbed, the sign of heat is taken to be positive and when heat is released, the sign of heat is taken to be negative.
Hence, the value of
 for the reaction will be -537 kJ
for the reaction will be -537 kJ