Answer: The concentration of
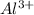 ions is 0.10 M and that of
ions is 0.10 M and that of
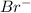 ions is 0.30 M
ions is 0.30 M
Step-by-step explanation:
We are given:
Concentration of
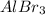 = 0.10 M
= 0.10 M
The chemical equation for the ionization of aluminium bromide follows:
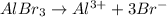
1 mole of aluminium bromide produces 1 mole of aluminium ions and 3 moles of bromide ions
So, concentration of aluminium ions = 0.10 M
Concentration of bromide ions =
![[(3* 0.1)]=0.3M](https://img.qammunity.org/2020/formulas/chemistry/college/emfk5b5cwuxvftynajhsmjk7lcsvd9ss6u.png)
Hence, the concentration of
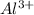 ions is 0.10 M and that of
ions is 0.10 M and that of
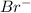 ions is 0.30 M
ions is 0.30 M