Answer:
28.05% is the mass percent of calcium carbonate in the sample.
Step-by-step explanation:
Suppose in 100 g of sample of impure calcium carbonate
Percentage of calcium in the sample = 11%
Mass of the calcium in the sample = 11% of 100 g =
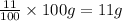
Moles of calcium =
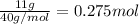
In 1 mole of calcium carbonate there are 1 mol of calcium .Then in 0.275 moles of calcium will be in :
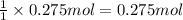 calcium carbonate
calcium carbonate
Mass of 0.275 moles of calcium carbonate :
0.275 mol × 102 g/mol =28.05 g
Percentage of calcium carbonate in the sample:
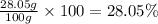
28.05% is the mass percent of calcium carbonate in the sample.