Answer:
(c)
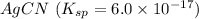
Step-by-step explanation:
The solubility product of a solid is the amount of solid dissociates into its respective ions in the solution. Thus more the value of the Ksp, the more is the salt soluble in the solvent.
So, Given that:-
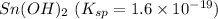
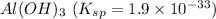
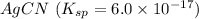
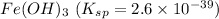
The salt having highest value of Ksp is AgCN. So, it is most soluble.