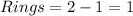Answer:
One ring is present.
Step-by-step explanation:
The degree of unsaturation or double bond equivalents(DBE) can be determined by using a formula. This formula gives us the number of rings and / pi bonds.
As mentioned in the problem the number of pi bond is one so if we calculate the DBE this will give us the number of rings by subtracting one from DBE.
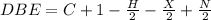
Where
C = number of carbon atoms
X = number of halogens
N = number of nitrogen
H = number of hydrogen
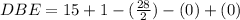
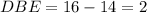
Thus the number of rings will be: