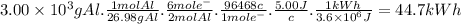Answer:
44.7 kWh
Step-by-step explanation:
Let's consider the reduction of Al₂O₃ to Al in the Bayer process.
6 e⁻ + 3 H₂O + Al₂O₃ → 2 Al + 6 OH⁻
We can establish the following relations:
- The molar mass of Al is 26.98 g/mol.
- 2 moles of Al are produced when 6 moles of e⁻ circulate.
- 1 mol of e⁻ has a charge of 96468 c (Faraday's constant).
- 1 V = 1 J/c
- 1 kWh = 3.6 × 10⁶ J
When the applied electromotive force is 5.00 V, the energy required to produce 3.00 kg (3.00 × 10³ g) of aluminum is: