To solve this problem it is necessary to apply the concepts related to the coefficient of performance. The coefficient of performance allows us to relate both the Heat and the Work done on a heat pump or cooling system to know the percentage of losses or input energy.
Mathematically your relationship can be expressed as
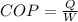
Where,
Q = Heat
W = Work
Our values are given as
COP =4.2
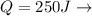 Cooling
Cooling
Replacing at our equation we have,
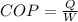
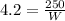
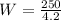
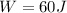
Therefore the work is 60J.