Step-by-step explanation:
It is known that according to mole concept, 1 mole of every substance contains
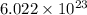 atoms.
atoms.
Therefore, mass of 1 mole of raindrops is as follows.
Mass of 1 mole raindrops = mass of 1 rain drop × Avogadro's number
=
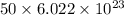
=
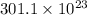 mg
mg
or, =
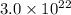 (as 1 mg = 0.001 g)
(as 1 mg = 0.001 g)
Therefore, mass of 1 mole of raindrops is
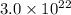 g.
g.
Now, the mass of Pacific ocean is calculated as follows.
Mass of Pacific ocean =
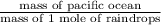
As 1 kg = 1000 g. So, mass of Pacific ocean will be
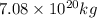 equal to
equal to
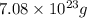 .
.
Hence, moles of raindrops will be calculated as follows.
Mass of Pacific ocean =
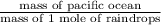
=
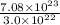
= 24 moles
Therefore, we can conclude that 24 moles of raindrops are in the Pacific Ocean.