Answer: The cell potential of the above reaction is 1.2 V
Step-by-step explanation:
The given chemical reaction follows:
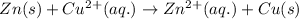
Oxidation half reaction:
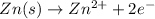
Reduction half reaction:
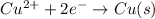
To calculate the EMF of the cell, we use the Nernst equation, which is:
![E_(cell)=E^o_(cell)-(0.059)/(n)\log ([Zn^(2+)])/([Cu^(2+)])](https://img.qammunity.org/2020/formulas/chemistry/college/z8hza8vgjawa024fk9s0qonfqhq53xhor7.png)
where,
 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V
 = standard electrode potential of the cell = 1.1 V
= standard electrode potential of the cell = 1.1 V
n = number of electrons exchanged = 2
![[Cu^(2+)]=1.0M](https://img.qammunity.org/2020/formulas/chemistry/college/1sh277nli18a2l92an96rgjz04cgj25en1.png)
![[Zn^(2+)]=1.0* 10^(-3)M](https://img.qammunity.org/2020/formulas/chemistry/college/isi35x8dumjkff38ork68qvau6bg81xhqw.png)
Putting values in above equation, we get:
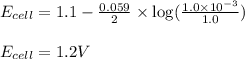
Hence, the cell potential of the above reaction is 1.2 V