Answer : The number of moles of oxygen present in a sample are 11.3 moles.
Explanation :
The given compound is,
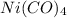
By the stoichiometry we can say that, 1 mole of of
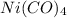 has 4 moles of CO.
has 4 moles of CO.
Or we can say that, 1 mole of of
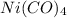 has 1 mole of nickel (Ni), 4 moles of carbon (C) and 4 moles of oxygen.
has 1 mole of nickel (Ni), 4 moles of carbon (C) and 4 moles of oxygen.
That means,
Number of moles of carbon = Number of moles of oxygen
As we are given that:
Number of moles of carbon = 11.3 moles
So, number of moles of oxygen = number of moles of carbon = 11.3 moles
Therefore, the number of moles of oxygen present in a sample are 11.3 moles.