Step-by-step explanation:
It is known that relation between osmotic pressure and temperature is as follows.
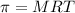
where,
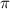 = osmotic pressure
= osmotic pressure
M = molarity of solution
R = gas constant
T = Temperature in Kelvin
The given data is as follows.
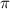 = 63.0 bar, T = (20 + 273) K = 293 K
= 63.0 bar, T = (20 + 273) K = 293 K
R = 0.0831 L bar/mol K
Therefore, putting the given data into the above formula as follows.
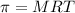
or, M =
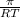
M =
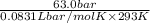
= 2.58 mol/L
Now,
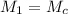 = 1.10 M, M = 2.58 M
= 1.10 M, M = 2.58 M
 = ?,
= ?,
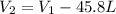
As,
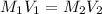
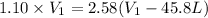
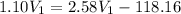
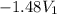 = -118.16
= -118.16
 = 79.83 L
= 79.83 L
Thus, we can conclude that the required volume of seawater is 79.83 L.