Answer:
E) less than 7
Step-by-step explanation:
pH is defined as the negative logarithm of the concentration of hydrogen ions.
Thus,
pH = - log [H⁺]
pH scale generally runs from 1 to 14 where pH = 7 represents neutral medium, pH < 7 represents acidic medium and pH > 7 represents basic medium.
Strong acids dissociate completely and when they react with base they form a salt of weak base and strong acid.
For example,
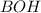 is a weak base and
is a weak base and
 is the hydrochloric acid.
is the hydrochloric acid.
So,
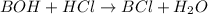
Thus, the salt is of weak base and strong acid which corresponds to pH less than 7.