Answer:
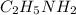
Step-by-step explanation:
Acids are the species which furnish hydrogen ions in the solution or is capable of forming bonds with electron pair species as they are electron deficient species.
Bases are the species which furnish hydroxide ions in the solution or is capable of forming bonds with electron deficient species as they are electron rich species.
The strongest base is also the weakest acid. Also, more the value of Kb, the strongest is the base and the weakest acid it will be.
Also, pKb is the negative logarithm of Kb value. Thus, Large value of Kb corresponds to least value of pKb.
So, The specie with the lowest value of pKb is the weakest acid.
Thus, out of the options, the lowest value is of 3.19 which is
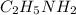 .
.