Step-by-step explanation:
It is known that electricity is the flow of ions or electrons. And, ionic substances are able to conduct electricity in aqueous state because they dissociate in ions in a solution.
Whereas covalent substances are unable to dissociate into ions in aqueous state. Hence, covalent substances do not conduct electricity.
Therefore, the given solid must be an ionic compound because it is able to conduct electricity in aqueous solution. Moreover, it is hard and brittle in nature so, it will exist in the form of
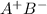 in solid state but in moleten state it will exist as
in solid state but in moleten state it will exist as
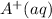 and
and
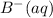 .
.
Thus, we can conclude that the given solid is an ionic solid.