Answer : The crown is not made of pure (100%) gold.
Explanation :
Formula used :
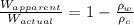
where,
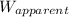 = apparent weight of the crown in water = 4.50 N
= apparent weight of the crown in water = 4.50 N
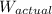 = actual weight = 5.00 N
= actual weight = 5.00 N
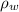 = density of water =
= density of water =
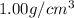
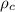 = density of crown = ?
= density of crown = ?
Now put all the given values in the above formula, we get:
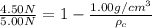
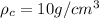
Density of crown < Density of gold
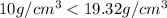
Thus, the crown is not made of pure (100%) gold.